What You Missed in 2025:
Spanning the full spectrum of analytical methods, from bioassays and molecular biology to physicochemical characterization, our comprehensive 2-track agenda delivers the technical depth and practical insights you need to move your programs forward.
Biotech, pharma, and leading service providers will present data-driven, case study-led sessions on analytical innovations for AAV-based gene therapies and the evolving landscape of novel capsids, LNP characterization, and gene transfer platforms. Whether you’re refining existing assays or developing analytics for new modalities, you’ll access comprehensive, in-depth, and current expertise to help shape the next generation of gene therapy products in 2025 and beyond.
Download the 2025 Brochure Here to Explore:
- 6 Interactive Workshops across 2 dedicated tracks
- 2 Comprehensive Content Tracks covering the full spectrum of analytical techniques
- 30+ Stellar Speakers from across leading biopharma
- Dedicated Networking Opportunities including speed networking, poster sessions, and informal meet-ups
What Was New for 2025?

Garner insights into potency assay design across modalities with case studies from Sanofi, Atsena Therapeutics, Regeneron, and more, uncovering how to build functionally relevant assays for AAV, LNP, mRNA, and gene-editing platforms.

Elevate regulatory preparedness by learning from Bristol Myers Squibb, Astellas, Spark Therapeutics, and others on navigating evolving FDA and EMA expectations for genome integrity, RC virus detection, and adventitious agent testing.

Navigate analytical bottlenecks such as full/empty capsid quantification, assay variability, and method transfer with practical guidance from REGENXBIO, Adverum Biotechnologies, and Eli Lilly on leveraging AUC, dPCR, and mass photometry.

Envision future-ready analytical strategies by benchmarking against innovations from Novartis, CSL, Sarepta, and more, addressing NGS method validation, stability testing, and platform-specific challenges beyond traditional AAV-based systems.
Companies that Have Already Confirmed their Attendance:
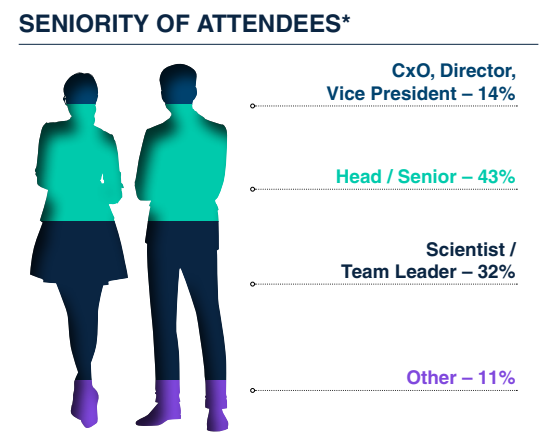
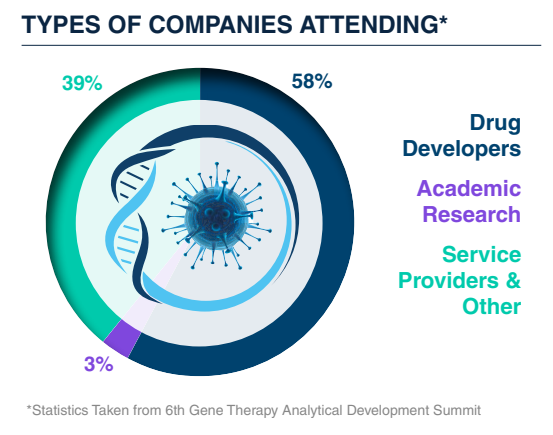
Who Will You Meet?
Join a focused community of analytical, assay, and process development leaders from the world’s top biotech and pharma companies. You'll connect with scientists, technical directors, and QC specialists who are driving innovation in gene therapy analytics–from molecular biology and bioassays to physicochemical methods and CMC strategy. Whether you're looking to benchmark your approach, exchange real-world experiences, or forge new collaborations, this is the place to meet the minds shaping the future of analytical development.